Calculate E for the titration of 50.00 mL of 0.100 M Ag+ when 50.00 mL of 0.150 M Cl− is added.Silver wire is the indicator electrode and the saturated Ag-AgCl electrode is the reference electrode.E = 0.197 V for the saturated Ag-AgCl electrode, 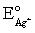 = 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
= 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
Definitions:
Decisions
Conclusions or resolutions reached after consideration.
Misguided
Description applied to actions, decisions, or beliefs that are based on incorrect assumptions, leading to unintended outcomes.
Similar Relationships
A concept in various fields that refers to relationships sharing common attributes, characteristics, or principles.
Subordinates
Individuals who are lower in rank or position within an organization and report to someone of higher rank.
Q4: Which of the following statements are true
Q4: If the measured potential of a half-cell
Q4: One liter of a pH 5.00 propionic
Q9: _ is the volume between the point
Q11: What you give up to obtain an
Q16: All of the following are true for
Q17: For EDTA titrations the titration reaction is
Q20: Which of the following statements regarding double
Q62: Describe significance of the leading and lagging
Q150: With the understanding that people respond to