A 50.00 mL aliquot of a water sample is reacted with excess potassium iodide in acidic solution to generate 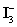 .Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the
.Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the 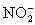 concentration in ppm? Assume solution density of 1.000 g/mL.
concentration in ppm? Assume solution density of 1.000 g/mL. 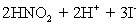 ⇋
⇋ 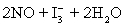
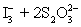 ⇋
⇋ 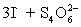
Definitions:
Producer Surplus
The difference between the amount producers are willing to accept for a good or service and the actual price they receive.
Below Equilibrium Price
A situation where the price of a good or service is set lower than the market equilibrium, often leading to a shortage.
Market Failure
A situation in which the allocation of goods and services by a free market is not efficient, often due to externalities, monopolies, or information asymmetries.
Efficient Outcome
An optimal allocation of resources where it is impossible to improve one party's position without worsening another's, often associated with maximized total welfare or utility in an economic context.
Q3: When extracting a sample with a liquid,the
Q4: One liter of a pH 5.00 propionic
Q11: _ is any chemical of interest.<br>A)Analyte<br>B)Species<br>C)Replicate<br>D)Aliquot<br>E)Bulk
Q16: A student must prepare 500.0 mL of
Q16: The part of a spectrophotometer that selects
Q57: With respect to the six stages of
Q64: With respect to American Express's change process,which
Q70: What is the structure and systems employed
Q198: According to Adam Smith,the success of decentralized
Q250: Suppose one county in Missouri decides it