A 50.00 mL aliquot of a water sample is reacted with excess potassium iodide in acidic solution to generate 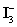 .Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the
.Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the 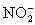 concentration in ppm? Assume solution density of 1.000 g/mL.
concentration in ppm? Assume solution density of 1.000 g/mL. 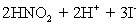 ⇋
⇋ 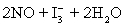
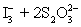 ⇋
⇋ 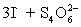
Definitions:
Sugar
A sweet-tasting soluble carbohydrate obtained primarily from sugarcane and sugar beet, widely used as a sweetener in food and drinks.
Surplus Production
The situation where the quantity of goods produced exceeds the quantity of goods consumed.
Government Policies
Decisions or plans made by a government that influence the behavior of society or sectors of the economy.
Farm Products
Agricultural goods produced through farming activities, including crops, livestock, and other raw materials.
Q2: The end point of some precipitation titrations
Q2: For redox titrations,the oxidation state of the
Q6: Doubling the number of calibration curve data
Q6: The van Deemter equation describes plate height
Q8: Which of the statements below is FALSE
Q8: For the reaction below,the oxidizing agent is_
Q10: The observed variance, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4000/.jpg" alt="The observed
Q20: The temperature program for a separation starts
Q153: Trade can make everyone better off except
Q192: To raise productivity,policymakers could<br>A) increase spending on