For the Acid-Base Indicator Bromothymol Blue,the Protonated Form,HBB,is Yellow and the Deprotonated
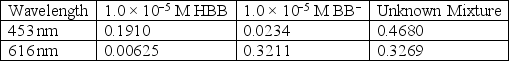
For the acid-base indicator bromothymol blue,the protonated form,HBB,is yellow and the deprotonated form,BB−,is blue.When both forms are present the indicator has a green color,the shade of green depends on the ratio of HBB:BB−.Use the information below to calculate the concentration of HBB and BB− for a green solution of bromothymol blue.
Definitions:
Fermentation
Anaerobic glucose-breakdown pathway that produces ATP without the use of electron transfer chains.
Pyruvate
A critical molecule in cellular metabolism that marks the end of glycolysis and the starting point for the Krebs cycle in aerobic respiration or fermentation in anaerobic conditions.
NAD+
Nicotinamide adenine dinucleotide, a coenzyme found in all living cells that is critical in metabolic processes, particularly in redox reactions.
NADH
Nicotinamide adenine dinucleotide (NADH) serves as an electron carrier, playing a crucial role in cellular energy production during the process of oxidative phosphorylation.
Q1: Which relationship is NOT correctly defined?<br>A)Work =
Q6: Which is NOT true of the flame
Q9: There are seven common strong acids.Which of
Q11: How many rings and double bonds are
Q12: A technician determines the concentration of calcium
Q12: Capillary electrophoresis separates two analytes with retention
Q15: An aqueous mixture that is 0.100 M
Q57: The self-interest of the participants in an
Q90: In the circular-flow diagram,households and firms are
Q262: The business cycle is measured by the<br>A)