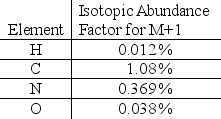
The mass spectrum of an unknown compound has a molecular ion peak at 194 m/z.The proposed chemical formula for the compound is C8H10N4O2.The M+1 peak has a relative abundance of 10.1%.Does the intensity of the M+1 peak support the proposed chemical formula?
Definitions:
Comparability
A quality of accounting information that allows users to analyze and compare financial data from different periods and entities in order to make informed decisions.
Capital Raised
The total amount of funds collected by a company from investors or financial markets, often used for business operations, expansion, or paying off debt.
Stock Options
Financial derivatives that give the holder the right, but not the obligation, to buy or sell a stock at a predetermined price within a specified time frame.
Diluted Earnings
Earnings per share calculated under the assumption that all convertible securities have been converted into common stock, providing a "worst case" scenario perspective on earnings per share.
Q3: The monochromator for a new instrument design
Q6: The end point for a titration can
Q6: _ is the titration of iodine produced
Q6: Which of the following are correct for
Q8: Which statement is NOT true for gas
Q12: Which is NOT true for ion mobility
Q55: Sarah buys and sells real estate.Two weeks
Q213: When calculating the cost of college,which of
Q215: In the circular-flow diagram,factors of production are
Q420: Refer to Figure 2-14.It is possible for