Copper has two naturally occurring isotopes with atomic masses of 62.9296 u ( 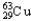 ) and 64.9278 u (
) and 64.9278 u ( 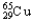 ) .The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?
) .The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?
Definitions:
Current Liabilities
Short-term financial obligations that are due to be paid within one year or within the business's operating cycle.
Q1: Choose the correct equilibrium constant expression for
Q7: Which of the following units is most
Q16: Which of the following statements is false?<br>A)Gas
Q18: Which of the following is the best
Q24: Convert 3.77 × 10<sup>8</sup> pL to mL,daL,and
Q24: CPAs must be independent to issue a
Q40: While there is no professional requirement to
Q43: Which of the following compounds are classified
Q47: Which of the following is the best
Q93: Which of the following is not a