Multiple Choice
Consider the following general structure. 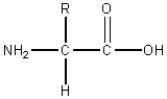 If the R group were HO-CH2-,this compound would be classified as:
If the R group were HO-CH2-,this compound would be classified as:
Definitions:
Related Questions
Q1: Which of the following is the best
Q2: 10.7 L of a gas at 1.75
Q18: Write the equation for the complete oxidation
Q19: When freshly cut sodium metal is exposed
Q27: When issuing a debt compliance letter,the auditor's
Q29: Consider the volumetric flask shown below.The volume
Q32: What is the name of the compound
Q44: A compound is analyzed and found to
Q44: According to Arrhenius theory,what is an acid?<br>A)A
Q44: The chemical symbol of argon is Ar.An