Consider the following table of relative strengths of oxidizing and reducing agents: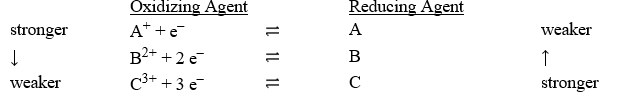
Which of the following is the correct net ionic equation for the redox reaction between A+ and C and the correct direction that is favored?
Definitions:
Surrogacy
An arrangement where a woman carries and delivers a child for another person or couple, often involving legal and contractual agreements.
Contracted Party
An entity or individual that has legally agreed to terms and conditions set forth in a contract with another party.
Ethical Dilemma
A situation in which a difficult choice has to be made between two or more morally correct actions that are in conflict.
Moral Principles
Fundamental beliefs about right and wrong that guide an individual's behavior and decision-making.
Q8: A water solution is considered acidic when
Q18: What is the oxidation number of chromium
Q20: Which of the following is the best
Q26: Which of the following does not correctly
Q27: Which of the following statements is incorrect?<br>A)Electronegativities
Q28: How many grams of calcium are in
Q35: A piece of potassium is placed in
Q40: Which of the following statements is incorrect?<br>A)Molecular
Q45: Calculate the normality of an unknown acid
Q55: Accruals that are based on estimated changes