A hydrochloric acid solution is standardized by titrating against solid sodium carbonate as shown in the following image.The HCl is in the buret and the sodium carbonate in the flask.  The equation is 2 HCl(aq) + Na2CO3(s) → 2 NaCl(aq) + H2O(
The equation is 2 HCl(aq) + Na2CO3(s) → 2 NaCl(aq) + H2O( 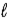 ) + CO2(g) .If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?
) + CO2(g) .If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?
Definitions:
Irreversible
Describing a process or action that cannot be undone or returned to its original state.
Content Dimensions
Aspects of a message that involve the actual information being communicated, distinct from the relationship dimensions.
Communicating Regret
is the expression of remorse or sorrow for actions or decisions that negatively impacted oneself or others.
Q1: Balance the following redox reaction in acidic
Q2: Which of the following statements is/are incorrect?<br>i.Nuclear
Q4: Which of the following compounds is a
Q5: What distinguishes natural radioactivity from induced radioactivity
Q15: Consider the following image. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3385/.jpg" alt="Consider
Q17: What is the electron pair geometry surrounding
Q19: The STP volume of an ideal gas
Q21: Choose the correct equilibrium constant expression for
Q26: A cylindrical gas chamber has a piston
Q47: In the Matrixx Initiatives v.Siracusano case,the Supreme