A hydrochloric acid solution is standardized by titrating against solid sodium carbonate as shown in the following image.The HCl is in the buret and the sodium carbonate in the flask.  The equation is 2 HCl(aq) + Na2CO3(s) → 2 NaCl(aq) + H2O(
The equation is 2 HCl(aq) + Na2CO3(s) → 2 NaCl(aq) + H2O( 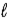 ) + CO2(g) .If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?
) + CO2(g) .If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?
Definitions:
Adjustment Message
Communication intended to address a complaint or request, aiming to rectify an issue and maintain customer satisfaction.
Sympathy Note
A written message expressing feelings of condolence and support for someone who has experienced loss or misfortune.
Communication Channel
The medium through which information is transmitted from sender to receiver, such as email, phone, or face-to-face.
Customer Complaints
Feedback from customers expressing dissatisfaction with a product or service.
Q2: Among the following bonds,which would be the
Q5: Bobek et al.found a disconnect between tax
Q5: What distinguishes natural radioactivity from induced radioactivity
Q8: Which of the following quantities is/are correctly
Q21: Which of the following compounds is/are (an)amine(s)?<br>i.CH<sub>3</sub>-NH<sub>2</sub><br>ii.(CH<sub>3</sub>)<sub>2</sub>-NH<br>iii.(CH<sub>3</sub>)<sub>3</sub>-N<br>iv.CH<sub>3</sub>-CO-NH<sub>2</sub><br>v.CH<sub>3</sub>(CH<sub>2</sub>)<sub>2</sub>-CO-NH<sub>2</sub><br>A)i
Q29: Balance the half-reaction in acidic solution and
Q30: Which of the following bases is not
Q40: The KPMG tax shelter case deals with:<br>A)The
Q41: The legal liability of the auditors in
Q45: Which of the following would be classified