1.25 g C6H5COOH is dissolved in water and the solution is titrated with 33.5 mL of a solution of NaOH of an unknown concentration.Calculate the normality of the NaOH for the reaction C6H5COOH(aq) + NaOH(aq) → C6H5COONa(aq) + H2O( 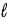 ) .
) .
Definitions:
Multiple T Tests
Statistical tests used to compare the means of two groups on several different variables or outcomes.
Grade Level
A step or stage in an education system that categorizes students based on age, development, or years of schooling.
Null Hypotheses
A hypothesis that there is no significant difference or effect and any observed difference is due to chance.
Research Hypotheses
Predictive assertions by scientists about the connection between multiple variables.
Q16: Which of the following statements is false?<br>A)Gas
Q16: Which of the following substances is amphoteric?<br>A)H<sub>2</sub>O(
Q23: A "particularized" allegation requires establishing:<br>A)Strong circumstantial evidence
Q26: What is the common reference conditions for
Q32: Consider the following figure. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3385/.jpg" alt="Consider
Q35: The pressure of the gas in a
Q40: Which of the following compounds is an
Q45: Consider the following structure.The central atom is
Q46: A gas has a density of 1.19
Q69: All of the following are examples of