The structural formula for glycerin is shown below.Compare the intermolecular forces in glycerin with those in n-hexane,C6H14,in which all carbons are in a continuous chain.Which of the following statements is true? 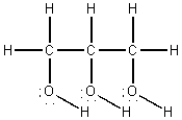
Definitions:
Separate From
To distinguish or differentiate between entities or concepts, making clear the distinction or division between them.
Buddhists
Adherents of Buddhism, a spiritual tradition focused on personal spiritual development and the attainment of a deep insight into the true nature of life.
First Cause
A philosophical and theological concept referring to the initial cause of the universe, often discussed in arguments for the existence of God or a prime mover.
Aquinas
A medieval philosopher and theologian, Thomas Aquinas is known for integrating Aristotelian philosophy with Christian doctrine, emphasizing ethics, natural law, and metaphysics.
Q3: Consider the following beaker which has had
Q8: The SEC's complaint in its case against
Q9: Why does the stability of a noble
Q11: The legal precedent that evolves from legal
Q17: A typical candy bar contains 281 food
Q22: Consider the particulate level representation of a
Q30: Which of the following is the best
Q35: What is the volume of 22 g
Q41: Explain what is meant by "quality of
Q43: Which of the following has a linear