Calculate the volume of hydrogen,measured at 19°C and 688 torr,that will be produced from 0.45 g of sodium in the reaction 2 Na(s) + 2 H2O( 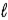 ) → 2 NaOH(aq) + H2(g) .
) → 2 NaOH(aq) + H2(g) .
Definitions:
Correlation Coefficients
A measure that determines the degree to which two variables' movements are associated.
Correlation Coefficient
A statistical measure that calculates the strength and direction of a linear relationship between two variables on a scatterplot.
Expected Return
The probable return on an investment, considering all possible outcomes and their probabilities, representing the average of all possible returns.
Beta
A measure of the volatility, or systematic risk, of a security or portfolio in comparison to the market as a whole.
Q1: Balance the following redox reaction in acidic
Q7: The framework of COSO's Enterprise Risk Management
Q8: What is the product of the reaction
Q26: Consider the following image representing an atom.
Q26: PCAOB inspections of U.S.audit firms operating in
Q28: A certain compound is a gas at
Q35: Consider a general comparison between acid-base and
Q38: Which of the following compounds is an
Q39: If 45.5 g of BaCl<sub>2</sub> is dissolved
Q41: Which of the following types of compounds