Calculate the volume of hydrogen,measured at 19°C and 688 torr,that will be produced from 0.45 g of sodium in the reaction 2 Na(s) + 2 H2O( 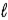 ) → 2 NaOH(aq) + H2(g) .
) → 2 NaOH(aq) + H2(g) .
Definitions:
Operating Income
Income from a company’s everyday business operations, calculated before taxes and interest.
Absorption Costing
A costing method that includes all manufacturing costs - direct materials, direct labor, and both variable and fixed overhead - as part of the cost of a produced unit.
Unit Product Cost
The total cost, including materials, labor, and overhead, divided by the number of units produced.
Year 2
Refers to the second year of a time-related context or sequence, not a standalone key term without additional context.
Q1: Which of these statements is true?<br>A)All Brønsted-Lowry
Q4: What volume of 0.372 M H<sub>2</sub>SO<sub>4</sub> contains
Q6: How does a phosphorus atom achieve an
Q6: Which of the following is the correct
Q6: Ethical leadership competence refers to:<br>A)Leaders ability to
Q9: Consider the segment of a polymer shown
Q24: What is the oxidizing agent in this
Q28: In the Loyalty and Fraud Reporting case,Ethan
Q38: Which of the following compounds is an
Q44: A substance that melts at 122°C is