Consider the following periodic table. 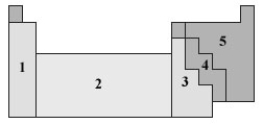 In what numbered section would the elements used in semiconductors be found?
In what numbered section would the elements used in semiconductors be found?
Definitions:
Melting Point
The temperature at which a solid becomes a liquid at atmospheric pressure.
Intermolecular Forces
Forces of attraction or repulsion which act between neighboring particles, such as atoms or molecules, affecting the physical properties of substances.
Bend
To cause something to change its shape by applying force without breaking.
Saturated
Describes a chemical compound, typically a fat, that contains no double bonds between carbon atoms, allowing it to hold a maximum number of hydrogen atoms.
Q6: The molar volume of a gas at
Q24: Which of the following gases should have
Q33: What is enterprise risk management (ERM)?<br>A)A process
Q37: Each of the following is a common
Q38: Under the Private Securities Litigation Reform Act
Q38: What is the molar mass of a
Q42: Explain the link between the opportunity to
Q44: Which of the following statements is/are incorrect?<br>i.Undissolved
Q49: Which of the following earnings management techniques
Q74: To whom does the CPA owe ultimate