Consider the following thermochemical equation: CaO(s) + H2O( 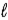 ) → Ca(OH) 2(s) ΔH = -66.5 kJ Which of the following statements is/are correct?
) → Ca(OH) 2(s) ΔH = -66.5 kJ Which of the following statements is/are correct?
i.The reaction is exothermic
ii.The reaction releases 66.5 kJ per gram of Ca(OH) 2(s) formed
iii.Alternatively,the ΔH term could be added to the product side of the equation
Definitions:
Pricing Policy
A strategic approach adopted by a company to set the cost of its products or services, often considering factors like market demand, production costs, and competition.
Linear Programming
A mathematical technique used for optimizing operations, focusing on achieving the best outcome in a model with linear relationships.
Constraint Lines
Limitations or bottlenecks within a production process that restrict the flow of production or services.
Objective Function
A mathematical equation used in optimization to represent the goal of an operation, often aiming to maximize or minimize some aspect of the system.
Q3: Consider the following beaker which has had
Q15: The auditor's responsibility with regard to illegal
Q22: An example of fraudulent financial statements is:<br>A)Misrepresentation
Q24: The heat of fusion of gold is
Q29: The main issue in the Valley View
Q31: In the reaction P<sub>4</sub> + 5 O<sub>2</sub>
Q33: Which of the following statements is true?<br>A)The
Q33: In the combustion of natural gas according
Q44: A substance that melts at 122°C is
Q53: The difference between errors in the financial