Consider the following thermochemical equation: CaO(s) + H2O( 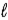 ) → Ca(OH) 2(s) ΔH = -66.5 kJ Which of the following statements is/are correct?
) → Ca(OH) 2(s) ΔH = -66.5 kJ Which of the following statements is/are correct?
i.The reaction is exothermic
ii.The reaction releases 66.5 kJ per gram of Ca(OH) 2(s) formed
iii.Alternatively,the ΔH term could be added to the product side of the equation
Definitions:
Chemoreceptors
Sensory receptors that respond to chemical stimuli, involved in taste, smell, and internal body conditions like oxygen and carbon dioxide levels.
Blood Concentration
The amount of a substance per unit volume of blood, indicating the level of various compounds or cells in the bloodstream.
Glucose
An uncomplicated glucose important for energy in living systems and a building block of numerous carbohydrate forms.
Somatic Sense
The sensory systems associated with the body that include touch, temperature, pain, and proprioception (sense of position and movement).
Q2: Identify the product(s)that is/are (a)compound(s)in the equation
Q5: Tommy Hubbs is the new controller of
Q14: In the third principal energy level,what is
Q20: Which of the following statements is/are correct?<br>i.A
Q30: Which of the following was not pointed
Q37: Each of the following is a common
Q41: What kind of molecular collisions will not
Q46: To be an ethical leader,executives must also:<br>A)Attend
Q60: Some critics claim the usefulness of the
Q61: Who distinguished between earnings manipulation and earnings