Multiple Choice
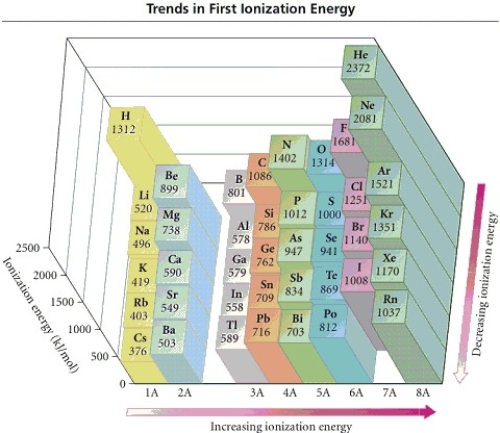
Refer to the figure.Place the following in order of increasing IE1.
N F As
Definitions:
Related Questions
Q2: Place the following gases in order of
Q23: Why is the ΔH<sub>vap</sub> higher than ΔH<sub>fus
Q71: How many of the following elements have
Q77: Which has the smallest dipole-dipole forces?<br>A)CH<sub>3</sub>Cl<sub> </sub><br>B)HBr<sub>
Q78: How many molecules of CO<sub>2</sub> are contained
Q78: Which of the following quantum numbers describes
Q87: How many photons are contained in a
Q92: A gas sample contains 0.33 atm of
Q113: Choose the diamagnetic species from below.<br>A)Sn<sup>2</sup>⁺<br>B)Br<br>C)P<br>D)Cr<br>E)None of
Q126: Nitrogen dioxide reacts with water to form