Vanadium crystallizes in a body-centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 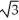
.
Definitions:
Middle 95%
Refers to the central portion of a distribution that contains 95% of the data, often used in the context of confidence intervals indicating where the true population parameter lies with a certain level of confidence.
Normal Model
A distribution of probability that exhibits symmetry around the mean, demonstrating that data points close to the mean occur more frequently than those far from it.
Volumes
Quantities of three-dimensional space enclosed within a boundary or the contents within a container, measured in cubic units.
Normal Model
A symmetric probability distribution around the mean, indicating that occurrences of data are more common close to the mean than far from it.
Q17: To make a 2.00 m solution,one could
Q22: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q25: What is the autoionization of water?
Q28: At 20°C,a 0.376 M aqueous solution of
Q29: Why do Li,Na,and K have similar chemical
Q45: Determine the value of K<sub>c</sub> for the
Q65: The reaction that occurs in a Breathalyzer,a
Q79: Which of the following elements can form
Q85: The osmotic pressure of a solution formed
Q94: A solution is prepared by dissolving 38.6