The enthalpy change for converting 1.00 mol of ice at -50.0°C to water at 70.0°C is 
The specific heats of ice,water,and steam are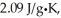
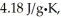
And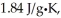
Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
Definitions:
Sustainability
Involves meeting the needs of the present without compromising the ability of future generations to meet their own needs, often focusing on environmental, economic, and social dimensions.
Business Strategies
Are comprehensive plans developed by organizations to achieve specific goals and objectives in a competitive market.
Organizations
Groups or entities comprising multiple individuals working collectively towards common goals or objectives, structured in a system of roles and responsibilities.
Stakeholder Assessment
A process to evaluate the interests, influence, and implications for all parties with a vested interest in a project or business decision.
Q15: Which of the following elements can form
Q16: Choose the best Lewis structure for XeI<sub>2</sub>.<br>A)
Q32: The elementary reaction<br>2NO<sub>2</sub>(g)→ 2NO(g)+ O<sub>2</sub>(g)<br>Is second order
Q46: Which of the following ions should have
Q66: What is the strongest type of intermolecular
Q68: Determine the K<sub>b</sub> for CN⁻ at 25°C.The
Q84: What is the hydronium ion concentration of
Q96: Using Lewis structures and formal charge,which of
Q98: Calculate the pH for an aqueous solution
Q105: Express the equilibrium constant for the following