The enthalpy change for converting 10.0 g of ice at -25.0°C to water at 80.0°C is ________ kJ.The specific heats of ice,water,and steam are 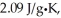
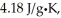 And
And 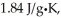 Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
Definitions:
Biome
One of the biosphere’s major communities, characterized in particular by certain climatic conditions and particular types of plants.
Temperature
A measure of the warmth or coldness of an environment or substance, typically expressed in degrees Celsius (°C), Fahrenheit (°F), or Kelvin (K).
Soil Conditions
The varying physical, chemical, and biological characteristics that influence the growth and health of plants.
Climate
The average weather conditions, including temperature, precipitation, and wind, in a region over a long period.
Q4: Which of the following acids is the
Q23: Identify the number of electron groups around
Q34: Calculate the hydroxide ion concentration in an
Q35: The Henry's law constant for helium gas
Q56: Which type of bonding does Sr form
Q62: Place the following in order of increasing
Q62: Choose the substance with the highest viscosity.<br>A)(CH<sub>3</sub>CH<sub>2</sub>)<sub>2</sub>CO<br>B)C<sub>2</sub>H<sub>4</sub>Cl<sub>2</sub><br>C)HOCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH<br>D)CF<sub>4</sub><br>E)C<sub>6</sub>H<sub>14</sub>
Q93: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6106/.jpg" alt=" Using the data
Q110: How many H<sup>-</sup> ions are around each
Q110: Determine the pH of a 0.22 M