Determine the radius of an Al atom (in pm) if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 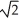
R.
Definitions:
Membership
The state or condition of being a member or part of an organization or group.
Public Policy
Principles and standards regarded by the legislative or judicial branches of government as being of fundamental concern to the state and its citizens.
Invalid Assignment
The transfer of rights or benefits under a contract that is not permitted or recognized by law, rendering it void.
Consideration
Something of value exchanged between parties in a contract, required for the agreement to be legally binding.
Q2: Which of the following represent the Lewis
Q9: Vanadium crystallizes in a body-centered cubic structure
Q24: Use Lewis theory to determine the chemical
Q28: What are the F-Po-F bond angles in
Q38: Give the change in condition to go
Q57: Place the following compounds in order of
Q60: The hybrid orbital set used by the
Q68: Calculate the wavelength of light associated with
Q78: Place the following substances in order of
Q111: Choose the bond below that is most