The enthalpy change for converting 1.00 mol of ice at -50.0°C to water at 70.0°C is 
The specific heats of ice,water,and steam are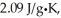
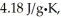
And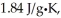
Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
Definitions:
Twenty-First Century
The current century, spanning from the year 2001 to 2100, characterized by rapid technological innovation and global interconnectedness.
Prison Population
The total number of individuals incarcerated in a country's prison system at any given time.
Prison-Industrial Complex
A term used to describe the overlapping interests of government and industry that use surveillance, policing, and imprisonment as solutions to economic, social, and political problems.
Q4: Which is expected to have the largest
Q7: For the reaction: N<sub>2</sub>(g)+ 2 O<sub>2</sub>(g)⇌ 2
Q25: Determine the rate law and the value
Q35: Determine the ammonia concentration of an aqueous
Q36: Define fusion.<br>A)The phase transition from solid to
Q58: Explain what the exponential factor in the
Q71: What is the hydroxide ion concentration and
Q112: Draw the best Lewis structure for CH<sub>3</sub><sup>+</sup>.What
Q120: In the best Lewis structure for BCl<sub>3</sub><sup>
Q122: What mass (in g)of NH<sub>3</sub> must be