Write a balanced reaction for which the following rate relationships are true.
Rate = -
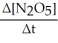 =
= 
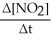 =
= 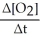
Definitions:
Global Licensing
A cross-border agreement that allows companies in different countries to use the properties (such as patents, trademarks, or technology) of another.
Turnkey Operation
A type of project that is constructed so that it can be sold or handed over to the buyer in a ready-to-use condition.
Chemical Factory
A facility where chemical processes are carried out, especially for manufacturing chemicals on a large scale.
Misused
Incorrect, inappropriate, or unsuitable use of something, often leading to harmful consequences.
Q24: Place the following in order of increasing
Q27: A solution with a hydroxide ion concentration
Q39: How many half-lives are required for the
Q41: The aquation of tris-(1,10-phenanthroline)iron(II)in acid solution takes
Q50: Identify the triprotic acid.<br>A)HNO<sub>3</sub><br>B)H<sub>3</sub>PO<sub>4</sub><br>C)H<sub>2</sub>SO<sub>3</sub><br>D)HClO<sub>4</sub><br>E)H<sub>2</sub>SO<sub>4</sub>
Q60: Choose the substance with the highest surface
Q62: The rate constant,k,for a first-order reaction is
Q70: Determine the rate law and the value
Q87: Find the percent ionization of a 0.337
Q126: Which one of the following statements is