Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25°C.(The equation is balanced. )
Pb(s) + Br2(l) → Pb2+(aq) + 2 Br⁻(aq) 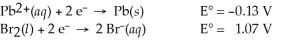
Definitions:
Tax Imposed
A compulsory financial charge or some other type of levy placed upon a taxpayer by a governmental organization.
Less Elastic
Describes a situation where the demand or supply for a good or service is less responsive to changes in price.
Supply Curve
A graphical representation that shows the relationship between the price of a good and the quantity supplied over a certain period of time, typically upward sloping.
Equilibrium Price
The monetary value at which the supply and demand of products in the market achieve parity.
Q3: A redox reaction has an E° cell
Q4: The graph in the figure shows the
Q28: Which of the following acids (listed with
Q34: The wavelength of the light from a
Q46: What is (0.674/0.74). Expressed to the correct
Q53: The x component of vector <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg"
Q55: What mass of aluminum can be plated
Q77: Two cars are traveling at the same
Q95: The K<sub>p</sub> for the reaction below is
Q105: The gas OF<sub>2</sub> can be produced from