Multiple Choice
Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25 °C.(The equation is balanced. )
2 K(s) + I2(s) → 2 K⁺(aq) + 2 I⁻(aq) 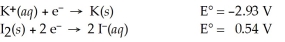
Definitions:
Related Questions
Q11: A 25.0-mL sample of 0.150 M butanoic
Q17: For which reaction will K<sub>p</sub> = K<sub>c</sub>?<br>A)S(s)+
Q22: Which of the following processes has a
Q27: A solution with a hydroxide ion concentration
Q39: Determine the identity of the daughter nuclide
Q62: Determine the molar solubility of BaF<sub>2 </sub>in
Q69: A velocity vector has components 36 m/s
Q72: Which of the following statements are true?<br>A)If
Q95: The number of significant figures in 0.01500
Q99: Which of the following acids is the