Determine which of the following pairs of reactants will result in a spontaneous reaction at 25°C. 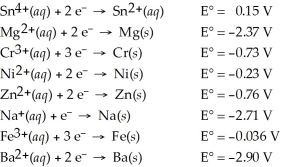
Definitions:
Plan-Do-Study-Act
A four-step, iterative method used for quality improvement in processes, involving planning, implementation, evaluation, and refinement.
Meta-Analysis
A statistical technique that combines the results of multiple scientific studies to obtain a more accurate effect size of an intervention or variable of interest.
Randomized Control Trials
A study design where participants are randomly allocated to receive one of several clinical interventions, one of which may be a standard treatment or a placebo, to determine the efficacy of interventions.
Systematic Review
A comprehensive summary of research literature on a specific topic, using systematic methods to collect and critically analyze studies.
Q2: Which of the following metals will dissolve
Q15: Nuclides below the valley of stability can
Q31: Give the criteria for a spontaneous reaction.<br>A)ΔG°
Q41: Which one of the following salts,when dissolved
Q55: In which of the following processes do
Q66: Which of the following processes has a
Q86: A marathon race is 26 mi and
Q87: What is the pH of a solution
Q103: Write a nuclear equation to describe the
Q122: A runner ran the marathon (approximately 42.0