Determine which of the following pairs of reactants will result in a spontaneous reaction at 25°C. 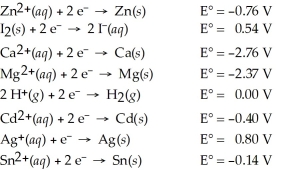
Definitions:
Compounded Semiannually
Interest calculation method where the interest is added to the principal sum every six months, thereby earning interest on interest.
Promissory Note
A financial instrument in which one party (the maker) promises in writing to pay a determinate sum of money to the other (the payee), either at a fixed or determinable future time or on demand.
Missing Interest Rate
The not specified or unidentified rate of interest in a financial context.
Real Compound Rate of Return
The annual rate of return on an investment, adjusted for inflation, that compounds over time.
Q7: Vector <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="Vector Has
Q21: To determine the height of a flagpole,Abby
Q42: Which of the following is not a
Q50: A geological sample is found to have
Q53: beta particle<br>A)<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6106/.jpg" alt="beta particle A)
Q59: Determine the pH of a 0.00598 M
Q64: If the velocity of an object is
Q81: To determine the height of a bridge
Q97: Calculate the pH of a solution formed
Q111: Abby throws a ball straight up and