Use the tabulated half-cell potentials below to calculate ΔG° for the following balanced redox reaction.
3 I2(s) + 2 Fe(s) → 2 Fe3+(aq) + 6 I⁻(aq) 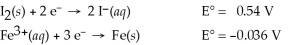
Definitions:
Debt Outstanding
The total amount of borrowed money that a company or government has not yet repaid to creditors or bondholders, including both principle and interest.
Basic Earning Power Ratio
A financial metric that measures a company's ability to generate earnings from its assets, regardless of financing.
HD
Typically refers to "High Definition," denoting a high level of detail in images or videos, but in a financial context, it could relate to "Home Depot" stock symbol or a concept not standardized across finance.
Operating Costs
Expenses associated with the day-to-day functions of a business, excluding the costs associated with producing goods or services.
Q3: A rock is projected upward from the
Q9: If the pK<sub>a</sub> of HCHO<sub>2</sub> is 3.74
Q53: An astronaut stands by the rim of
Q76: Calculate the ΔG°<sub>rxn</sub> using the following information.<br>2
Q81: Which of the following is the weakest
Q81: To determine the height of a bridge
Q88: Determine the identity of the daughter nuclide
Q90: An airplane needs to reach a forward
Q98: The length and width of a rectangle
Q112: Use the tabulated half-cell potentials below to