Use the tabulated half-cell potentials below to calculate the equilibrium constant (K) for the following balanced redox reaction at 25°C.
3 I2(s) + 2 Fe(s) → 2 Fe3+(aq) + 6 I⁻(aq) 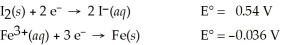
Definitions:
Unpaid Seller
A seller who has not received payment for goods supplied.
Cover
A legal remedy in contract law where the aggrieved party purchases goods or services, similar to those agreed upon, from a different source due to the original party's failure to comply.
Incidental Damages
Expenses or losses that occur as a direct result of a breach of contract, distinct from direct damages.
Consequential Damages
A type of damages that arise not directly from a wrongful act but from the results of the act, which may not have been foreseeable.
Q4: Determine the redox reaction represented by the
Q17: Consider the following reaction at constant P.Use
Q18: What element is being oxidized in the
Q29: What is the sum of 1.53 +
Q32: What is the pH of a solution
Q40: An equilibrium mixture of CO,O<sub>2</sub> and CO<sub>2</sub>
Q51: Use the tabulated half-cell potentials below to
Q61: What percentage of a radioactive substance remains
Q93: Cyclohexane,C<sub>6</sub>H<sub>12</sub>,undergoes a molecular rearrangement in the presence
Q114: Which of the following is a Lewis