Use the tabulated half-cell potentials below to calculate the equilibrium constant (K) for the following balanced redox reaction at 25°C.
Pb2+(aq) + Cu(s) → Pb(s) + Cu2+(aq) 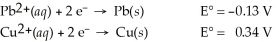
Definitions:
Financial Reports
Official records that outline the financial activities, status, and health of a business, organization, or individual.
Purchasing Decisions
The process involved in identifying and procuring goods and services based on need, quality, and price considerations.
In-House Attorneys
Lawyers employed directly by corporations or organizations to provide legal services and advice internally, as opposed to outside law firms.
Chief Executive Officers
Top executives responsible for the overall management, strategic direction, and decision-making in an organization or company.
Q7: In a room where g = 9.81
Q30: In addition to a beta particle,what is
Q35: You walk <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="You walk
Q55: A toy rocket is launched vertically from
Q87: Define reaction quotient.
Q95: A jet plane is launched from a
Q104: A car is traveling with a constant
Q105: pH < 7<br>A)half-way to equivalence point of
Q120: Determine the pH of a 0.227 M
Q121: A solution is prepared by dissolving 0.23