Calculate the cell potential for the following reaction that takes place in an electrochemical cell at 25°C.
Sn(s) ∣ Sn2+(aq,0.022 M)  Ag+(aq,2.7 M) ∣ Ag(s)
Ag+(aq,2.7 M) ∣ Ag(s) 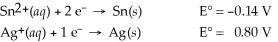
Definitions:
Depolarization
A reduction in the membrane potential of a cell, such as a nerve or muscle cell, making it more positive and closer to the threshold for triggering an action potential.
Taste Buds
Sensory organs located on the tongue that allow us to perceive different flavors such as sweet, salty, sour, and bitter.
Olfactory Neurons
Specialized nerve cells responsible for the sense of smell, located in the olfactory epithelium of the nasal cavity.
Mechanoreceptors
Sensory receptors that respond to mechanical pressures or distortions, such as touch, pressure, vibration, and sound.
Q3: The volume of a 10-mL test tube
Q14: Vector <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="Vector Has
Q33: A 100.0 mL sample of 0.18 M
Q36: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q51: The following reaction represents what nuclear process?
Q60: Based on the following information,<br>Cl<sub>2</sub>(g)+ 2 e<sup>-</sup>
Q65: Determine the equilibrium constant for the following
Q69: Identify the change in state that does
Q129: Determine the molar solubility of AgI in
Q147: A 1.0 L buffer solution is 0.050