Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25°C.(The equation is balanced. )
3 Cl2(g) + 2 Fe(s) → 6 Cl⁻(aq) + 2 Fe3+(aq) 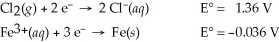
Definitions:
Efficient Process
A procedure characterized by minimal waste and maximum productivity, effectively achieving its intended result with the least amount of time and resources.
Chemiosmosis
The movement of ions across a semipermeable membrane, down their electrochemical gradient, which is used by cells to generate ATP during processes like photosynthesis and cellular respiration.
Glycolysis
The metabolic pathway that breaks down glucose into pyruvate, releasing energy and pyruvic acid, the first step in cellular respiration.
Citric Acid Cycle
A set of chemical transformations that enable aerobic entities to produce energy, through the oxidation of acetate from carbohydrates, fats, and proteins into carbon dioxide.
Q7: At a certain time,the average size of
Q7: Calculate ΔS°<sub>rxn</sub> for the following reaction.The S°
Q26: Potassium-40 decays to argon-40 with a half-life
Q40: How long must a constant current of
Q46: Which one of the following would be
Q47: The number of significant figures in 0.040
Q54: Carbon-11 is used in medical imaging.The half-life
Q69: Calculate P [NO]eq,if P [NOCl]<sub>eq</sub> = 0.33
Q70: Identify the missing particle in the following
Q112: A 100.0 mL sample of 0.10 M