Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25°C.(The equation is balanced. )
Pb(s) + Br2(l) → Pb2+(aq) + 2 Br⁻(aq) 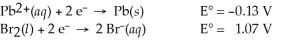
Definitions:
Competitors Merge
The process in which two or more competing businesses combine into a single entity, potentially altering market dynamics and competitive landscapes.
Book Publishing
The activity of making literature, information, or other content available to the public for sale or distribution.
Pulp and Paper Mill
An industrial facility where wood pulp is produced and then used to manufacture paper products, ranging from newspapers to packaging materials.
Vertical Merger
A vertical merger is the combination of two or more companies at different stages of production in the same industry, aiming to increase efficiency or market power.
Q12: Calculate the pOH of a solution that
Q27: Nuclides above the valley of stability can
Q27: An auto manufacturer advertises that their car
Q37: A 7.0 × 10<sup>-3</sup> M aqueous solution
Q43: Place the following in order of increasing
Q44: How many seconds are required to produce
Q48: Vector <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="Vector Has
Q53: The x component of vector <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg"
Q61: Balance the following redox reaction if it
Q69: Calculate P [NO]eq,if P [NOCl]<sub>eq</sub> = 0.33