Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25°C.(The equation is balanced. )
Sn(s) + 2 Ag⁺(aq) → Sn2+(aq) + 2 Ag(s) 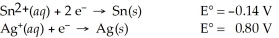
Definitions:
Non-Invasive Technique
A method or procedure that does not require entering the body or breaking the skin, used for diagnostic or therapeutic purposes.
Stereotaxic Apparatus
Surgical instrument that permits a researcher or neurosurgeon to target a specific part of the brain.
Subcortical Lesions
Damage or abnormalities in the brain regions located below the cerebral cortex, potentially affecting movement, memory, and emotion regulation.
Aspiration Lesion
A neurosurgical procedure where targeted brain tissue is removed or destroyed using suction, often employed in research to study brain function by observing behavior changes post-lesion.
Q4: Which of the following acids is the
Q19: Identify the missing particle in the following
Q22: Determine the identity of the daughter nuclide
Q44: A 100.0 mL sample of 0.20 M
Q47: The number of significant figures in 0.040
Q66: Which of the following processes has a
Q68: A racing car accelerates uniformly from rest
Q85: How many of the following are weak
Q93: A car is moving with a constant
Q95: Place the following in order of decreasing