Use the tabulated half-cell potentials below to calculate the equilibrium constant (K) for the following balanced redox reaction at 25°C.
2 Al(s) + 3 Mg2+(aq) → 2 Al3+(aq) + 3 Mg(s) 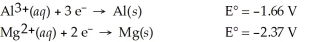
Definitions:
Monopsonist
A market situation where there is only one buyer for many sellers, giving the single buyer significant control over prices and terms of purchase.
Competitive Labor Market
A market scenario where numerous buyers (employers) and sellers (workers) freely interact to determine the wages and employment conditions without significant restrictions or monopoly power.
Profit-Maximizing Level
The production output level at which a business achieves the highest possible profit, where marginal cost equals marginal revenue.
Total Labor Cost
The complete expenditure incurred by an employer for the compensation of employees, including wages, benefits, and taxes.
Q12: Which of the following processes has a
Q15: A bicyclist starts a timed race at
Q20: Identify the missing particle in the following
Q53: An astronaut stands by the rim of
Q58: Determine ΔG°<sub>rxn</sub> using the following information.<br>FeO(s)+ CO(g)→
Q69: A 100.0 mL sample of 0.10 M
Q78: Determine the cell notation for the redox
Q85: Describe an alpha particle.<br>A)electromagnetic radiation<br>B)two neutrons and
Q85: Which of the following metal cations is
Q102: Which particle has the highest ionizing power?<br>A)alpha