Use the tabulated half-cell potentials below to calculate the equilibrium constant (K) for the following balanced redox reaction at 25°C.
3 I2(s) + 2 Fe(s) → 2 Fe3+(aq) + 6 I⁻(aq) 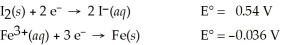
Definitions:
Introduction Section
The initial part of a document, presentation, or research paper that outlines the purpose, scope, and objectives of the content that follows.
Solution
A means or method of solving a problem or dealing with a challenging situation.
Persuasive Approach
A strategy aimed at influencing someone's attitudes, intentions, beliefs, or behaviors through reasoning or appeal to emotion.
Direct Approach
A communication strategy that involves presenting the main point or purpose at the beginning, followed by supporting details.
Q23: Describe what changes occur during beta decay.<br>A)The
Q27: How many electrons are transferred in the
Q31: What is the equilibrium constant (K<sub>c</sub>)for the
Q35: You leave on a 549-mi Trip in
Q37: What is the pH of a buffer
Q57: Which of the following bases is the
Q70: Identify the missing particle in the following
Q74: Determine the identity of the daughter nuclide
Q93: Which of the following is a Br∅nsted-Lowry
Q102: Determine the equilibrium constant for the following