Use the tabulated half-cell potentials below to calculate the equilibrium constant (K) for the following balanced redox reaction at 25°C.
Pb2+(aq) + Cu(s) → Pb(s) + Cu2+(aq) 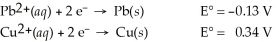
Definitions:
OSHA
The Occupational Safety and Health Administration, a federal agency of the United States responsible for ensuring safe and healthy working conditions.
Split Rims
Split rims are a type of vehicle wheel construction, consisting of multiple pieces that are bolted together, making tire changes easier but also bearing certain safety concerns.
Tire Disposal
The process of properly discarding used or unwanted tires, which involves recycling or repurposing to prevent environmental harm.
Radial Tires
Tires constructed with the cord plies arranged at a 90-degree angle to the direction of travel, providing improved strength, stability, and fuel efficiency.
Q6: If you are 5'10'' tall,what is your
Q29: What is the conjugate base of H<sub>2</sub>PO<sub>4</sub><sup>⁻</sup>?<br>A)HPO<sub>4</sub><sup>2-</sup><br>B)PO<sub>4</sub><sup>3-</sup><br>C)H<sub>3</sub>PO<sub>4</sub><br>D)H<sub>3</sub>O<sup>+</sup><br>E)OH<sup>⁻</sup>
Q31: Which one of the following salts,when dissolved
Q44: The number 0.00325 × 10<sup>-8</sup> cm can
Q48: Which nuclide below is most likely to
Q64: A swimmer heading directly across a river
Q95: Place the following in order of decreasing
Q98: Balance the following redox reaction if it
Q124: A car is moving with a speed
Q136: What is the pH of a solution