Calculate the cell potential for the following reaction that takes place in an electrochemical cell at 25°C.
Mg(s) ∣ Mg2+(aq,2.74 M) ∣∣ Cu2+(aq,0.0033 M) ∣ Cu(s) 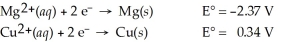
Definitions:
Tax Revenue
The income that the government receives from taxpayers, including both individuals and businesses, used to fund public services and infrastructure.
Multiplier Effect
The relative rise in total income resulting from an additional expenditure.
Chain Reaction
A sequence of reactions where a reactive product or by-product causes additional reactions to take place.
Recessionary Gap
The gap that occurs when an economy's actual output is less than its potential output, indicating underutilized resources.
Q5: James and John dive from an overhang
Q6: Vector <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="Vector Has
Q18: What element is being oxidized in the
Q60: An object moving in the +x direction
Q65: What is undergoing reduction in the redox
Q83: Identify the weak diprotic acid.<br>A)HNO<sub>3</sub><br>B)H<sub>3</sub>PO<sub>4</sub><br>C)H<sub>2</sub>SO<sub>3</sub><br>D)HClO<sub>4</sub><br>E)H<sub>2</sub>SO<sub>4</sub>
Q84: Balance the following redox reaction if it
Q99: Which of the following statements is true?<br>A)Entropy
Q100: Which one of the following will form
Q127: What is the hydronium ion concentration in