A container of 114.0 g of water is heated using 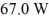
Of power,with perfect efficiency.How long will it take to raise the temperature of the water from 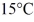
To 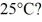
The specific heat capacity of the container is negligible,and the specific heat capacity of water is 4.186 × 103 J/kg ∙ C.
Definitions:
Flexible Leadership Theory
A management perspective that suggests successful leaders are those who can adapt their style to the demands of different situations.
Direct Behavior
Actions that are deliberate and intentional, often aiming to influence or guide others towards achieving specific goals or outcomes.
Leadership Succession
The process of identifying and preparing new leaders to take over leadership roles within an organization.
Chief Executives
Top-ranking corporate officers who have the ultimate decision-making responsibility and oversee the entire operation of an organization.
Q15: Two tiny particles having charges of +7.00
Q25: A rectangular box of negligible mass measures
Q61: When a current flows through an ionic
Q63: The figure shows two tiny 5.0-g spheres
Q63: In a section of horizontal pipe with
Q71: By how many decibels does the intensity
Q74: A glass beaker of unknown mass contains
Q84: The process shown on the pV diagram
Q99: An object weighs 7.84 N when it
Q208: A resistor,an uncharged capacitor,a dc voltage source,and