A container of 114.0 g of water is heated using 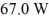
Of power,with perfect efficiency.How long will it take to raise the temperature of the water from 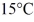
To 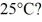
The specific heat capacity of the container is negligible,and the specific heat capacity of water is 4.186 × 103 J/kg ∙ C.
Definitions:
Q3: An object has a volume of 4.0
Q18: Suppose that a rigid aluminum wire were
Q19: A cinder block of mass m =
Q25: A simple pendulum and a mass oscillating
Q38: A proton moves 0.10 m along the
Q39: Doubling the capacitance of a capacitor that
Q56: A 1200-kg car is being raised with
Q59: A certain automobile tire has a volume
Q62: A sealed 87- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="A sealed
Q93: A parallel-plate capacitor consists of two parallel,square