In a flask,114.0 g of water is heated using 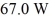
Of power,with perfect efficiency.How long will it take to raise the temperature of the water from 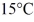
To 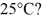
The specific heat of water is 4186 J/kg ∙ K.
Definitions:
Specific Objective
A clearly defined and measurable goal that an organization or individual aims to achieve.
Resources
Assets or inputs used to produce goods and services, including natural, human, and capital resources.
Purchase Method
An accounting method used to consolidate the financial statements of two companies when one acquires another.
Book Value
The net value of a company's assets minus its liabilities, often used to determine the company's equity value on its balance sheet.
Q2: A 0.50-kg object is attached to an
Q6: A glass flask has a volume of
Q21: A hypothetical planet has a mass of
Q36: A 4.2-L flask of ideal neon gas
Q39: If the amplitude of the motion of
Q68: An electron and a proton are released
Q75: A pipe that is 0.92 m long
Q75: If an ideal gas molecule has a
Q94: On his honeymoon,James Joule attempted to explore
Q101: How much work is needed to carry