How much heat must be added to a 8.0-kg block of ice at -8°C to change it to water at 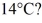
The specific heat of ice is 2050 J/kg ∙ °C,the specific heat of water is 4186 J/kg ∙ °C,the latent heat of fusion of ice is 334,000 J/kg,and 1 cal = 4.186 J.
Definitions:
Injection
A method of delivering medication or other substances into the body, often through a syringe and needle.
Title VII
A portion of the Civil Rights Act of 1964 that prohibits employment discrimination based on race, color, religion, sex, or national origin.
Civil Rights Act
Legislation in the United States aimed at ending discrimination based on race, color, religion, sex, or national origin.
Medical Care
Professional health services provided by doctors, nurses, and other health professionals to diagnose, treat, and prevent disease and injury.
Q4: What is the net power radiated by
Q24: An astronaut goes out for a "space-walk"
Q28: Solar houses use a variety of energy
Q52: The figure shows a graph of the
Q54: An 920-g piece of iron at 100°C
Q59: If the result of your calculation of
Q71: Which of the following is a false
Q74: A glass beaker of unknown mass contains
Q75: The coefficient of linear expansion for aluminum
Q97: A concrete wall of a cold storage