A substance has a melting point of 20°C and a heat of fusion of 3.4 × 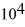
J/kg.The boiling point is 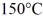
And the heat of vaporization is 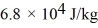
At a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid) ,1000 J/kg ∙ K (liquid) ,and 400 J/kg ∙ K (gaseous) .How much heat is required to raise the temperature of 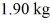
Of this substance from 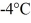
To 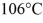
At a pressure of one atmosphere?
Definitions:
Recruiting Websites
Online platforms or services that facilitate the matching of employers with potential employees.
Traditional Recruitment
The classic method of filling job vacancies through advertisements, job postings, or hiring agencies, without the extensive use of modern technology.
Job Satisfaction
The level of contentment and fulfillment a person feels towards their job, influenced by various factors such as work environment, nature of the job, and interpersonal relationships.
Early Job Satisfaction
The level of contentment and fulfillment an individual feels in their job early in their career.
Q4: When a heavy metal block is supported
Q16: For the graph shown in the figure,what
Q20: A balloon originally has a volume of
Q47: Substance A has a density of 3
Q47: A fluid in an insulated,flexible bottle is
Q55: When a container of water is placed
Q67: If a quantity you calculated has units
Q76: A parallel-plate capacitor is connected to a
Q77: Two tuning forks have frequencies of 440
Q201: A 2.0-μF capacitor and a 4.0-μF capacitor