A substance has a melting point of 20°C and a heat of fusion of 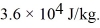
The boiling point is 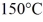
And the heat of vaporization is 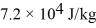
At a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid) ,1000 J/kg ∙ K (liquid) ,and 400 J/kg ∙ K (gaseous) .How much heat is given up by 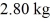
Of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
Definitions:
Push Strategy
A marketing approach that involves taking the product directly to the customer via whatever means to ensure the customer is aware of your brand at the point of purchase.
Supply Chain
A system of organizations, people, activities, information, and resources involved in moving a product or service from supplier to customer.
Physical Distribution
The activities concerned with the efficient movement of products from the producer to consumers, including storage and transportation.
Supply Chain Management
The oversight of materials, information, and finances as they move from supplier to manufacturer to wholesaler to retailer to consumer.
Q10: Find the speed of an ocean wave
Q19: If 50 g of lead (of specific
Q21: The absolute temperature of an ideal gas
Q32: An architect is interested in estimating the
Q57: A metal bar is 20 cm long
Q62: A solid steel column is 4.0 m
Q66: A pendulum of length L is suspended
Q72: If,with steady state heat flow established,you double
Q84: Water flowing through a cylindrical pipe suddenly
Q99: A blacksmith is flattening a steel plate