At what temperature would the root mean square speed of oxygen molecules,O2,be 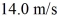
If oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.
Definitions:
Punishment
A penalty or negative consequence imposed for disobedience, wrongdoing, or other undesirable behavior.
Positive Reinforcement
A technique in behavior management that involves rewarding a desired behavior, thereby increasing the likelihood that the behavior will occur again.
Negative Reinforcement
A method of increasing the probability of a behavior by removing or avoiding a negative condition or stimulus following the desired behavior.
Extinction
In psychology, the diminishing of a conditioned response, occurring when the reinforcement no longer follows the conditioned stimulus; in biology, the end or dying out of a species.
Q15: The capacitive network shown in the figure
Q21: Three identical 3.0-µC charges are placed at
Q40: An ideal Carnot engine has an efficiency
Q50: A heat pump with a performance coefficient
Q67: A hollow steel ball of diameter 3.0
Q71: A proton that is initially at rest
Q72: What is the magnitude of a the
Q92: A dielectric material such as paper is
Q95: A piano wire of linear mass density
Q138: Two in-phase loudspeakers that emit sound with