How much heat must be added to a 8.0-kg block of ice at -8°C to change it to water at 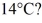
The specific heat of ice is 2050 J/kg ∙ °C,the specific heat of water is 4186 J/kg ∙ °C,the latent heat of fusion of ice is 334,000 J/kg,and 1 cal = 4.186 J.
Definitions:
Over-optimism
Over-optimism refers to the tendency of individuals to hold an unrealistically positive view of the future or the outcomes of events, which can affect decision-making processes.
Unrealistic Belief
A conviction or expectation that does not align with actual facts or evidence, often based on incorrect assumptions or misinformation.
Slope
A measure of the steepness, incline, or grade of a line, calculated as the ratio of the vertical change to the horizontal change between two points.
Budget Line
A graphical representation of all possible combinations of two goods that can be purchased with a given income and prices.
Q7: If a charge generator builds a negative
Q8: Point charges +4.00 μC and +2.00 μC
Q12: Suppose you wanted to hold up an
Q14: Ideal incompressible water is flowing in a
Q21: Two taut strings of identical mass and
Q40: How many grams of ethanol (density 0.80
Q49: An ideal Carnot engine operates between a
Q55: What is the resistance of a 0.100-kW
Q63: Two in-phase loudspeakers are 3.0 m apart.They
Q67: If a quantity you calculated has units