A substance has a melting point of 20°C and a heat of fusion of 3.4 × 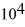
J/kg.The boiling point is 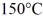
And the heat of vaporization is 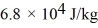
At a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid) ,1000 J/kg ∙ K (liquid) ,and 400 J/kg ∙ K (gaseous) .How much heat is required to raise the temperature of 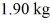
Of this substance from 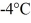
To 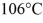
At a pressure of one atmosphere?
Definitions:
Short-run Phillips Curve
A visual diagram illustrating the short-term inverse correlation between inflation rates and unemployment rates.
Policymakers
Individuals or groups responsible for making and implementing policies, especially in government and legislative contexts.
Natural-rate Hypothesis
The theory suggesting that there is a specific level of unemployment that exists in an economy that is not eliminated by monetary policy in the long run.
Favorable Supply Shock
An unexpected event that suddenly increases the supply of a product or service, resulting in decreased prices and increased quantity.
Q48: Two metal rods,one silver and the other
Q59: A certain automobile tire has a volume
Q60: A tiny particle carries a charge of
Q66: For an ideal gas,<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="For
Q71: A 0.40- m<sup>3</sup>. Gas tank holds 7.0
Q78: An object attached to an ideal spring
Q89: Consider two copper wires of equal length.One
Q111: You double your distance from a sound
Q113: A proton is accelerated from rest through
Q141: A certain metal wire has a cross-sectional