A substance has a melting point of 20°C and a heat of fusion of 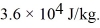
The boiling point is 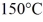
And the heat of vaporization is 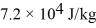
At a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid) ,1000 J/kg ∙ K (liquid) ,and 400 J/kg ∙ K (gaseous) .How much heat is given up by 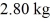
Of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
Definitions:
Q4: A simple pendulum has a period T
Q10: A 10-L flask and a 1-L flask
Q10: Find the speed of an ocean wave
Q11: During an isochoric process,the internal (thermal)energy of
Q22: When 5.00 A is flowing through an
Q47: The figure shows a group of three
Q60: The temperature of an ideal gas in
Q64: How much heat is required to raise
Q70: In an adiabatic compression,200 J of work
Q72: An ideal air-filled parallel-plate capacitor consists of