A rigid container is filled with 4.0 mol of air with CV = 2.5R.How much does the internal (thermal) energy of the air change if its temperature rises from 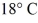
To 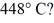
(R = 8.31 J/mol ∙ K)
Definitions:
Disease
A particular abnormal condition that negatively affects the structure or function of all or part of an organism, and that is not due to any external injury.
Holistic Medical Practitioner
A healthcare professional who treats patients by considering their physical, emotional, social, and spiritual needs.
Positive Outlook
A mental attitude characterized by hope and confidence in success and a positive future.
Sociological Issue
A problem or challenge that affects individuals or groups within a society, often requiring collective solutions or interventions.
Q6: A 100-kg person sits on a 5-kg
Q7: A sample of an ideal gas is
Q54: The ocean thermal energy conversion project uses
Q65: An ideal air-filled parallel-plate capacitor with horizontal
Q74: A hydrogen nucleus,which has a charge +e,is
Q87: A thermally isolated system is made up
Q87: A tube of mercury with resistivity 9.84
Q129: One of the harmonics of a column
Q149: The length of a certain wire is
Q180: A group of 1.0-μF,2.0-μF,and 3.0-μF capacitors is